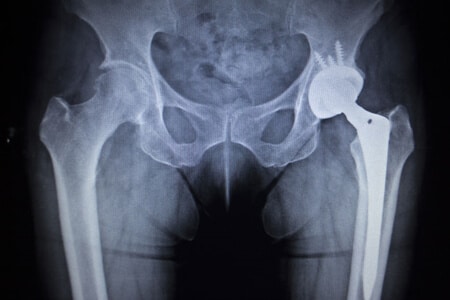
This case involves a 70 year-old woman with a history of rheumatoid arthritis who used the Zimmerman hip device after she fell at her home and dislocated her hip. After assessing her condition, the doctor decided a hip replacement was her best option. The operation went well and the patient was allowed to leave after four days. However, she soon started to experience pain and swelling in her hip area but did not inform her doctor until the pain became severe. A later evaluation showed she had developed metallosis since receiving the Zimmer hip replacement. She subsequently questioned the safety and regulations used for the Zimmer hip.
Question(s) For Expert Witness
- 1. Have you reviewed cases involving the Zimmer hip device? If yes, please explain.
-
2. Have you ever been involved in the approval process for this type of device or similar devices? If yes, please explain.
Expert Witness Response E-007633
I have a PhD in Biomedical Engineering and am a former FDA Lead Medical Device Reviewer. I used to be an instructor for Medical Device Regulation classes at a major state university. I have reviewed cases like this involving Zimmer, as well as similar issues with other manufacturers. I was a lead medical device reviewer at the FDA in Cardiovascular (predominantly Class III products, and some Class II). I also served as the FDA representative for the ASTM F04 task group, charged with the development of new testing standards for all medical devices.
Contact this expert witness
Related Posts
This case involves a patient in his late 30s who was undergoing treatment for fibromyalgia. He was prescribed multiple pain medications throughout the course of his treatment. One of the medications prescribed was a high dosage of Fentanyl. The patient…
This case involves a client whose personal property was seized by the IRS. On the date of the incident in question, the target of the IRS seizure – a high net worth individual – was away from his home when…